Long-lasting Salt Bridges Provide the Anchoring Mechanism of Oncogenic Kirsten Rat Sarcoma Proteins at Cell Membranes
Dec 30, 2020
Huixia Lu and Jordi Marti from the SIMCOM research group of the Physics department publish an article on the anchoring mechanism to the cell membrane of certain proteins relevant to oncological studies
Oncology is a branch of medicine that studies benign and malignant tumors; the latter known as cancers. Oncology begins with prevention, aimed at reducing the incidence of cancer, continuing with care for the sick and, finally, it is completed with research, which deals with the study of all the elements that intervene in the development of malignant diseases and its treatments.
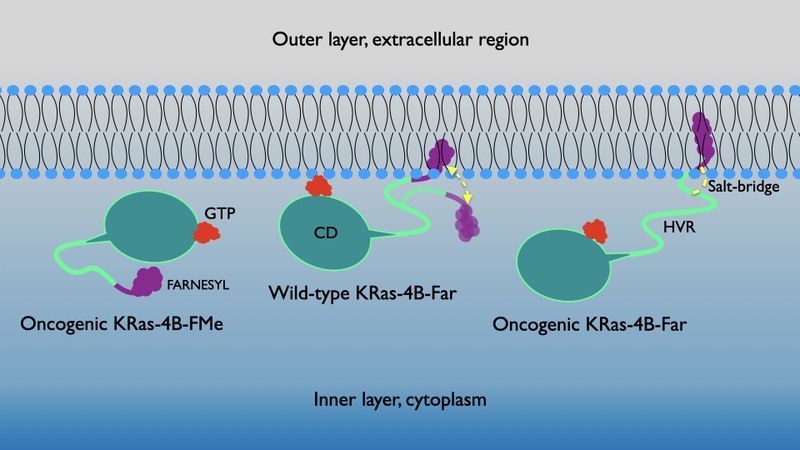
Ras proteins, together with the gene that bears the same name, are a set of very important molecular switch-regulators in a wide variety of cellular signal transmission routes that control different phenomena such as the integrity of the cytoskeleton, proliferation, cell differentiation, adhesion and migration and apoptosis. Both the gene and related Ras proteins are often altered in a wide variety of malignant tumors, causing increased invasiveness and metastasis, and decreased apoptosis. In particular, Ras proteins are directly involved in cancers of the lung, colon, and pancreas. These proteins function as binary switches between guanosine-diphosphatase and guanosine-triphosphatase (GDP-GTP) and are capable of regulating the cytoplasmic signaling networks that control various cellular processes, playing an essential role in the signaling of transduction pathways involved in growth. , differentiation and cell survival, in such a way that it is believed that excessive signaling is what can cause the growth of tumors. One of the most difficult challenges to face is the design of selective mutations that lead to efficient therapeutic strategies. In this work, the GTP-bound protein "Kirsten rat sarcoma" (KRas-4B) with a farnesylated tail and mutated to the amino acid G12 has been simulated at the interface of an anionic cell membrane model (DOPC / DOPS / cholesterol). Using molecular dynamics and metadynamic simulations, a specific long-term salt bridge between the farnesyl group and the hypervariable region of the protein has been identified as the main mechanism responsible for the binding of KRas-4B to the cell membrane. Free energy hypersurfaces have made it possible to characterize their global and local minima, which reveal the main transition pathways between anchored and non-anchored states.
The results have been recently published in the article entitled: "Long-lasting salt bridges provide the anchoring mechanism of oncogenic KRas-4B proteins to cell membranes" in The Journal of Physical Chemistry Letters. The reference in the article is:
H.Lu and J.Martí, J. Phys. Chem. Lett. 2020, 11, 9938−9945.
Share: